Answer:
The correct answer is -
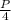 .
.
Step-by-step explanation:
By the ideal gas equation is PV=nRT, the relation between pressure and volume can be drive if the temperature remains constant:
At constant temperature, for a fixed amount of gas, P∝
so the pressure is inversely proportional to volume. Therefore, if the volume is increased in this condition the pressure will decrease in the same manner and A decrease in the volume will lead to an increase in pressure.
Volume increased here = four times = 4V
pressure is P will decrease four times = P/4