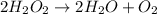Step-by-step explanation:
Hydrogen peroxide (H2O2) when decomposes forms water and oxygen in a reaction as shown below
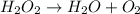
However, this reaction is not completely balanced as number of oxygen atoms on reactant and product sides are not same
So, the complete balance equation looks like