You would need to add approximately 57 grams more of
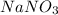 to have a saturated solution at 40°C.
to have a saturated solution at 40°C.
The solubility of sodium nitrate (
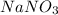 ) at 40°C is approximately 102 g per 100 g of water.
) at 40°C is approximately 102 g per 100 g of water.
If you have already dissolved 45 g of NaNO3 in 100 g of water, the amount of
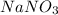 you would need to add to reach saturation at 40°C would be:
you would need to add to reach saturation at 40°C would be:
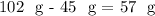
So, you would need to add approximately 57 grams more of
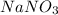 to have a saturated solution at 40°C.
to have a saturated solution at 40°C.