Answer:
The new volume is 8.64 L.
Step-by-step explanation:
Charles's Law consists of the relationship that exists between the volume and the temperature of a certain quantity of ideal gas, which is maintained at a constant pressure. For a given sum of gas at constant pressure, as the temperature increases, the volume of the gas increases and as the temperature decreases, the volume of the gas decreases.
In summary, Charles's law is a law that says that when the amount of gas and pressure are kept constant, the quotient that exists between the volume and the temperature will always have the same value:
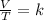
Being an initial state 1 that modifies its conditions until a final state 2, it is fulfilled:
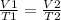
In this case:
- V1= 8 L
- T1= 326 K
- V2= ?
- T2= 352 K
Replacing:
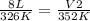
Solving:
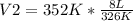
V2= 8.64 L
The new volume is 8.64 L.