Answer:
1. Molecular weight = 333.3 g/mol
2. Strenght = 2222.2 mg/L
Step-by-step explanation:
First, we need to find molarity (M) from normality (N) as follows:
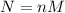
Where n is the number of OH⁻ = 2
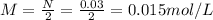
Now, when the H₃X solution is neutralized with Ca(OH)₂ we have:
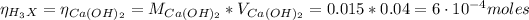
Hence, the molecular weight (MW) of H₃X is:
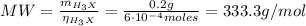
Finally, we can calculate the strength (S) of this solution in ppm as follows:
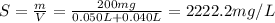
I hope it helps you!