Answer:
NaCl + AgF → NaF + AgCl
Step-by-step explanation:
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products.
From all the reactions given ,
- 2Na + Cl₂ → 2NaCl is an example of combination reaction because two or more reactants (Na & Cl₂) react with each other to form a single product (NaCl)
- H₂SO₃ → H₂O + SO₂ is an example of decomposition reaction because a single reactant (H₂SO₃) breaks down into two or more products (H₂O & SO₂).
- 2K + 2H₂O → 2KOH + H₂ is an example of displacement reaction because a highly reactive element (K) displaces a least reactive element (H) from its compound (H₂O).
- NaCl + AgF → NaF + AgCl is an example of double replacement reaction because there's an exchange between Cations (
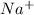 &
&
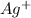 ) and Anions (
) and Anions (
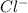 &
&
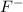 ).
).