Answer:
5.52 g
Step-by-step explanation:
First we convert the given masses of both reactants into moles, using their respective molar masses:
- 6.30 g NH₃ ÷ 17 g/mol = 0.370 mol NH₃
- 1.80 g O₂ ÷ 32 g/mol = 0.056 mol O₂
Now we calculate with how many NH₃ moles would 0.056 O₂ moles react, using the stoichiometric coefficients:
- 0.056 mol O₂ *
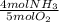 = 0.045 mol NH₃
= 0.045 mol NH₃
As there more NH₃ moles than required, NH₃ is the excess reactant.
Then we calculate how many NH₃ moles remained without reacting:
- 0.370 mol NH₃ - 0.045 mol NH₃ = 0.325 mol NH₃
Finally we convert NH₃ moles into grams:
- 0.325 mol NH₃ * 17 g/mol = 5.52 g