Answer: The theoretical yield of potassium chloride is 0.596 grams and the % Yield of potassium chloride is 83.9%
Step-by-step explanation:
To calculate the moles :
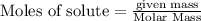
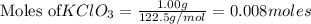
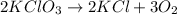
According to stoichiometry :
2 moles of
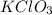 produce= 2 moles of
produce= 2 moles of
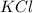
Thus 0.008 moles of
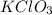 will produce=
will produce=
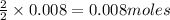 of
of
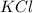
Theoretical yield of
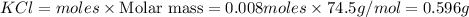
% yield =
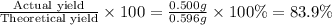
The theoretical yield of potassium chloride is 0.596 grams and the % Yield of potassium chloride is 83.9%