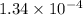The given question is incomplete. The complete question is:
A chemist prepares a solution of mercury(I) chloride
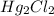 by weighing out 0.537 mg of mercury(I) chloride into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/dL of the chemist's mercury(I) chloride solution. Round your answer to 3 significant digits
by weighing out 0.537 mg of mercury(I) chloride into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/dL of the chemist's mercury(I) chloride solution. Round your answer to 3 significant digits
Answer: The concentration in g/dL of the chemist's mercury(I) chloride solution is
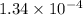
Step-by-step explanation:
Concentration of a solution is defined as how many grams of solute in=s dissolved in a particular amount of solvent.
Given : mass of mercury (I) chloride = 0.537 mg =
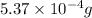
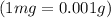
Volume of solution = 400 ml = 4 dL (1ml=0.01dL)
Thus concentration of mercury (I) chloride in g/dL is =
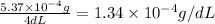
The concentration in g/dL of the chemist's mercury(I) chloride solution is