Answer:
The percentage by mass of benzene in the solution is approximately 0.2%
Step-by-step explanation:
The given parameters are;
The mass of the benzene solute dissolved in the gasoline solvent, m₁ = 1.56 g
The total volume of the benzene gasoline solution made, V = 998.44 mL
The density of gasoline, ρ = 0.7489 g/mL
Mass, m = Density, ρ × Volume, V
∴ The mass of gasoline in the 998.44 mL, solution = 0.7489 g/mL × 998.44 mL = 747.731716 g
The total mass of the solution = The mass of the benzene in the solution + The mass of the gasoline in the solution
∴ The total mass of the solution = 747.731716 g + 1.5 g = 749.231716 g
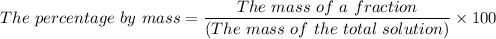
The percentage by mass of benzene in the solution = (1.5 g/749.231716 g)×100 ≈ 0.2% by mass.