Answer:
V₂ = 457.49 mL
Step-by-step explanation:
Given that,
Initial volume,
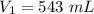
Initial temperature,
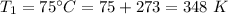
Final temperature,
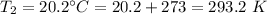
We need to find the final volume of the gas. The relation between the volume and the temperature of the gas is given by :
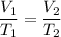
Put the respected values,
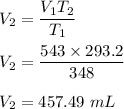
So, the final volume of the gas is equal to 457.49 mL.