Answer:
Step-by-step explanation:
Percent yield is a ratio of the actual yield to the theoretical yield. It is found using this formula:
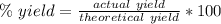
The actual yield is 12 liters, because that was actually produced in the lab.
The theoretical yield is 20 liters, because that was the expected yield.
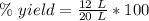
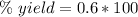
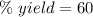
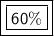
For this reaction, the percent yield is 60%.