Answer:
75% of Cl-35 and 25% of Cl-37
Step-by-step explanation:
Equation to find the mass number of an element. repeat the brackets for however many isotopes it has.
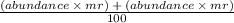
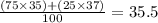
You can try guess it because 35.5 is closer to 35 than 37 so it has a higher abundance of Cl-35