Answer: The value of the equilibrium constant for this reaction is 2.7
Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as
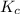
For the given chemical reaction:
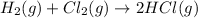
The expression for
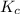 is written as:
is written as:
![K_c=([HCl]^2)/([H_2]^1[I_2]^1)](https://img.qammunity.org/2022/formulas/chemistry/college/iiq4km52j2tj55lwxws5sfk5wmshqegwk2.png)
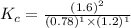
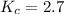
Thus the value of the equilibrium constant for this reaction is 2.7