Answer:
The electrical energy consumed in boiling the water is 0.0788 kWh
Step-by-step explanation:
Given;
mass of the water, m = 600 g = 0.6 kg
power rating of the kettle, P = 1500 W = 1.5 kW
specific heat capacity of water, c = 4,200 J/kg⁰C
density of water, = 1000 kg/m³
time taken to boil the water, t = 3 mins + 9 s = (3 x 60s) + 9 s = 180 s + 9 s = 189 s =
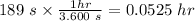
The electrical energy consumed in boiling the water is calculated as;
E = P x t
E = 1.5 kW x 0.0525 hr
E = 0.0788 kWh
Therefore, the electrical energy consumed in boiling the water is 0.0788 kWh