Answer:
M = 0.0678 M
Step-by-step explanation:
Hello there!
In this case, since the molarity of a solution is obtained by dividing the moles by the volume, it is firstly necessary to compute the moles of magnesium chloride in 0.968 g, via its molar mass, as shown below:
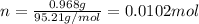
Next, since 150.0 mL in liters is 0.1500 L, according to the appropriate units, the resulting molarity is:
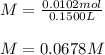
Best regards!