Answer:
The new temperature is 19.072 K.
Step-by-step explanation:
Charles's Law consists of the relationship that exists between the volume and the temperature of a certain quantity of ideal gas, which is maintained at a constant pressure. This law says that as the temperature increases, the volume of the gas increases and as the temperature decreases, the volume of the gas decreases because the temperature is directly related to the energy of the movement of the gas molecules.
In summary, Charles's law is a law that says that when the amount of gas and pressure are kept constant, the quotient that exists between the volume and the temperature will always have the same value:
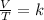
Assuming that a certain volume of gas V1 that is at a temperature T1 at the beginning of the experiment varies up to a volume of gas V2, then the temperature will change to T2, and it will be true:
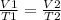
In this case:
- V1= 500 mL
- T1= 25 C= 298 K
- V2= 32 mL
- T2= ?
Replacing:
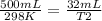
Solving:
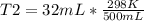
T2= 19.072 K
The new temperature is 19.072 K.