Answer:
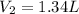
Step-by-step explanation:
Hello there!
In this case, since this problem is describing how pressure changes as a function of volume and vice versa, it is possible recall the Boyle's law as shown below:
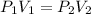
Whereas we are asked to compute the volume when the change is pressure is performed (V₂); thus, we proceed as follows:
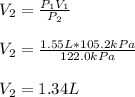
Best regards!