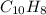Answer:
Step-by-step explanation:
Molecular formula can be calculated by finding the ratio of the molecular molar mass to the empirical molar mass, then multiplying the subscripts of the empirical molar mass by that ratio.
The empirical molar mass is 5(12.01) + 4(1.01) = 64.09g
The molecular molar mass is 128.16g
128.16/64.09 = 2
So the molecular formula is