Answer:

Step-by-step explanation:
Hello there!
In this case, it is possible to propose an energy balance in order to illustrate how the heat released by the reaction is absorbed by the water:
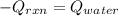
Thus, since the heat released by the reaction is -112 kJ (-112000 J), it is possible to define the hear absorbed by the water in terms of mass, specific heat and temperature change:
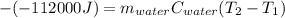
In such a way, it is possible to define the final temperature as shown below:
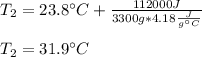
Best regards!