Answer: The total pressure, in atm is 2.62
b. The mole fraction of argon in the container is 0.67.
Step-by-step explanation:
a) According to Dalton's law, the total pressure is the sum of individual pressures.
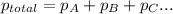
Given :
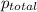 =total pressure of gases = ?
=total pressure of gases = ?
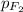 = partial pressure of fluorine = 0.85 atm
= partial pressure of fluorine = 0.85 atm
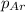 = partial pressure of Argon = 1350 torr = 1.77 atm ( 760 torr = 1atm)
= partial pressure of Argon = 1350 torr = 1.77 atm ( 760 torr = 1atm)
putting in the values we get:
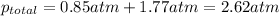
b) According to Raoult's law, the partial pressure of a component at a given temperature is equal to the mole fraction of that component multiplied by the total pressure.
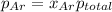
where, x = mole fraction
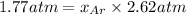
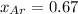
The mole fraction of argon in the container is 0.67.