Answer:
ΔV = -2.1 L
Step-by-step explanation:
To solve this exercise we can use the ideal gas equation for two points
PV = nRT
P₁V₁ = P₂ V₂
where point 1 is on the surface and point 2 is at the desired depth,
V₂ =
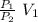
let's calculate
V₂ = (
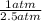 ) 3.5 L
) 3.5 L
V₂ = 1.4 L
this is the new volume, the change in volume is
ΔV = V₂ -V₁
ΔV = 1.4-3.5
ΔV = -2.1 L