Answer:
Step-by-step explanation:
The principal reason why these statements are equivalent and identical is that there can be a progressional flow of heat from a hot object item to a cold object because of this entropy is increasing and expanding Δg < 0 but stream flow from cold to hot is nonspontaneous as Δg > 0.
e.g
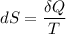
As found in heat engines and refrigerators.