Answer:
You would have 1.96 moles.
Step-by-step explanation:
You are given 608 grams of calcium phosphate Ca₃(PO₄)₂.
Molar mass (M) is the amount of mass that a substance contains in one mole. This substance can be an element or a compound.
Since the molar mass of the compound Ca₃(PO₄)₂ is 310 g / mole, and taking into account the definition of molar mass, you can apply the following rule of three: if 310 grams of Ca₃(PO₄)₂ are contained in 1 mole, 608 grams of the compound in how many moles are present?
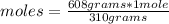
moles= 1.96
You would have 1.96 moles.