Answer:
a)
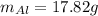
b)
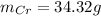
c)
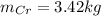
d)
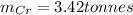
Step-by-step explanation:
The reaction is:
Cr₂O₃(s) + 2Al(s) → 2Cr(s) + Al₂O₃(s)
a) To find the Al mass needed to react with 50 g of Cr₂O₃, we need to calculate the number of moles of Cr₂O₃:
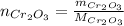
Where:
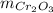 : is the mass = 50 g
: is the mass = 50 g
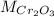 : is the molar mass = 2*52+3*16 = 152 g/mol
: is the molar mass = 2*52+3*16 = 152 g/mol
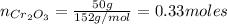
Now, the estoichiometric relation between Cr₂O₃ and Al is 1:2, so:
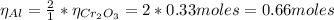
Hence, the mass of Al is:
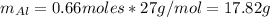
b) The stoichiometric relation from Cr₂O₃ and Cr is 1:2, hence:
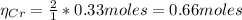
Thus, the mass of Cr is:
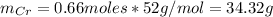
c) The number of moles of Cr₂O₃ with a mass of 5 kg is:
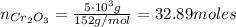
So, the mass of Cr is:
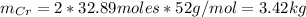
d) The mass of Cr produced from 5 tonnes of Cr₂O₃ is:
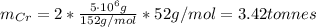
I hope it helps you!