Answer:
Q = 219.07 J
Step-by-step explanation:
Given that,
Mass of water, m = 15.4 g
The temperature changes from 22.2°C. to 25.6 °C.
The specific heat of water, c = 4.184 J/g °C
We need to find the heat needed to change in temperature. The formula is given by :
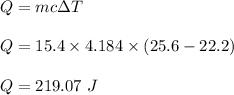
So, 219.07 J of heat is needed.