Answer:
m = 0.0035 m.
Step-by-step explanation:
Hello there!
In this case, since the formula for the computation of the molality is:
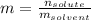
We can first compute the moles of solute, K3PO4 by using its molar mass:
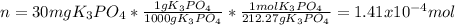
Next, since the volume of water is 40.0 mL and its density is 1.00 g/mL we infer we have the same grams (40.0 g). Thus, we obtain the following molality by making sure we use the mass of water in kilograms (0.04000kg):
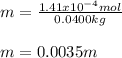
In molal units (m=mol/kg).
Best regards!