Answer:
3.57 M
Step-by-step explanation:
The reaction that takes place is:
- 2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
First we calculate how many NaOH moles reacted, using the given concentration and volume:
- 1.90 M * 38.70 mL = 73.53 mmol NaOH
Then we convert NaOH moles into H₂SO₄ moles, using the stoichiometric coefficients:
- 73.53 mmol NaOH *
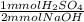 = 36.765 mmol H₂SO₄
= 36.765 mmol H₂SO₄
Finally we calculate the concentration of H₂SO₄:
- 36.765 mmol H₂SO₄ / 10.30 mL = 3.57 M