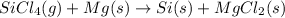Answer: Silicon tetrachloride (g) + Mg (s)
 Silicon (s) + Magnesium chloride (s)
Silicon (s) + Magnesium chloride (s)
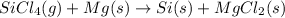
Step-by-step explanation:
The word equation for Solid silicon and solid magnesium chloride form wen silicon tetrachloride gas reacts with magnesium metal :
Silicon tetrachloride (g) + Mg (s)
 Silicon (s) + Magnesium chloride (s)
Silicon (s) + Magnesium chloride (s)
The skeletal equation where law of conservation of mass is not followed will be: