Answer:

Step-by-step explanation:
At STP (standard temperature and pressure), all gases have a volume of 22.4 liters per mole.
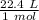
Multiply by the given number of moles, 4.0
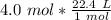
The moles will cancel out. The 1 in the denominator can be ignored, so this becomes a simple multiplication problem.
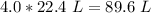
4.0 moles of a gas at STP have a volume of 89.6 liters.