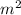Answer:
1.
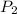 = 8 x
= 8 x
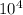 Pa
Pa
2. P = 1.5 x
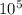 N/
N/
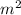
Step-by-step explanation:
1. From Boyles' law;
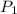
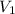 =
=
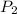
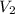
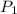 = 1 x
= 1 x
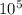 Pa
Pa
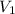 = 9.6
= 9.6
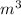
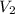 = 12
= 12
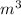
Thus,
1 x
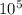 x 9.6 =
x 9.6 =
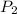 x 12
x 12
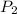 =
=
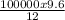
= 80000
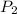 = 8 x
= 8 x
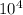 Pa
Pa
2. Pressure, P = ρhg
where: ρ is the density of the fluid, h is the height/ depth and g is the acceleration due to gravity (9.8 m/
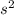 ).
).
Thus,
P = 1020 x 15 x 9.8
= 149940
P = 1.5 x
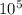 N/
N/