Answer:
B) 16 g
Step-by-step explanation:
First we convert 4 moles of O₂ into moles of H₂, using the stoichiometric coefficients of the balanced reaction:
- 4 mol O₂ *
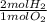 = 8 mol H₂
= 8 mol H₂
Finally we convert 8 moles of H₂ into grams, using its molar mass:
- 8 mol H₂ * 2 g/mol = 16 g
Thus, the correct answer is option B).