Answer: Iodine and Nitrogen
Step-by-step explanation:
An ionic bond is formed when an element completely transfers its valence electron to another element. The element which donates the electron is known as electropositive element or the metal and the element which accepts the electrons is known as electronegative element or non metal.
Calcium (Ca) being a metal will donate electrons to form a cation called as
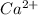 . Thus it will bond ionically to non metals which can form anions.
. Thus it will bond ionically to non metals which can form anions.
Iodine can form
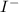 and Nitrogen can form
and Nitrogen can form
![]N^(3-)](https://img.qammunity.org/2022/formulas/chemistry/college/i8pza3yqxcmi60c8uem203y001p9bim7t5.png) , thus forming
, thus forming
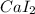 and
and
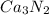 respectively.
respectively.