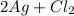Answer:
The given equation
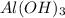 →
→
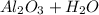 is the type of decomposition reaction.
is the type of decomposition reaction.
Step-by-step explanation:
DECOMPOSITION REACTION-: A decomposition reaction occurs when a single compound decomposes into two or more elements or new compounds. These reactions take place when energy is supplied in the form of heat, electricity, or light, which allows the material to break down into simpler substances.
The most common form of decomposition reaction is -
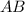 →
→
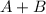
TYPES OF DECOMPOSITION REACTION IS -
- THERMAL DECOMPOSITION REACTION -: Thermolysis, or thermal decomposition, is a chemical decomposition caused by heat. The temperature at which a material chemically decomposes is known as its decomposition temperature. Heat is needed to sever chemical bonds in the compound being decomposed, so the reaction is normally endothermic.
Example- As calcium carbonate (limestone or chalk) is heated, it breaks down into calcium oxide and carbon dioxide. The following is the chemical reaction:
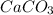 →
→
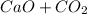
- ELECTROLYTIC DECOMPOSITION REACTION -: An electrolytic reaction is a chemical reaction in which electricity is passed through the aqueous solution of the reactant, resulting in the breakdown of the reactant into the substance.
Example -: When electricity is passed through an aqueous solution of sodium chlorine (NaCl), sodium and chlorine are created.
NaCl → Na + Cl
- PHOTOLYTIC DECOMPOSITION REACTION -: Light or photons are used to break down the reactants into many components in this form of reaction. The photo decomposition reaction is the decomposition reaction that occurs as a result of the action of light. Photolysis is another name for these reactions. In these reactions, light absorption excites electrons, which causes bonds to break and products to form.
Example - As silver chloride is exposed to light, it decomposes into silver metal and chlorine.
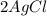 →
→