Answer:
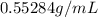
Step-by-step explanation:
Hello there!
In this case, given the by-mass percent of ethanol:
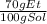
It is possible to compute the density by using its molar mass to find the grams in one liter of solution:
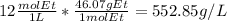
However, by taking the g/mL we obtain:
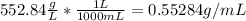
Which is the density of the solution.
Best regards!