Answer:
d. 149 ⁰C.
Step-by-step explanation:
Given;
mass of the block of ice, m = 2 kg
specific heat capacity of the ice, C = 2090 J/(kgK)
initial temperature of the ice, t₁ = -90 ⁰C
heat added to the ice, H = 1,000,000 J
let the final temperature of the ice = t₂
The final temperature of the ice after adding the heat is calculated as follows;
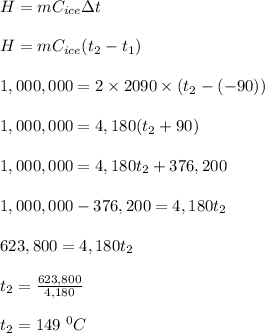
Therefore, the new temperature of the water is 149 ⁰C.