Answer:
Retention factor or
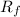 value is defined as the ratio of distance traveled by the unknown to the distance traveled by the solvent front.
value is defined as the ratio of distance traveled by the unknown to the distance traveled by the solvent front.
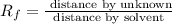
Putting in the values we get:
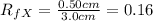
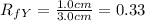
As the
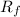 value of Y is more than X , it follows that Y compound will be retained less strongly than the compound X. Thus Y is more polar than X.
value of Y is more than X , it follows that Y compound will be retained less strongly than the compound X. Thus Y is more polar than X.