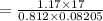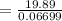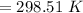Answer:
The temperature is "298.51 K".
Step-by-step explanation:
The given values are:
Density of ammonia,
d = 0.812 g/L
Pressure,
P = 1.17 atm
Mass:
M =
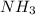
=
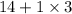
=
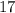
As we know,
⇒

∴
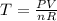
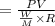
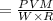
By putting the value of "W", get
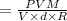
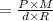
On substituting the values in the above equation, we get