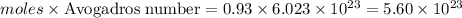The given question is incomplete. The complete question is:
Determine how many carbon dioxide molecules are produced if molecules of water are produced
Answer:
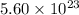 molecules of carbon dioxide are produced
molecules of carbon dioxide are produced
Step-by-step explanation:
The balanced chemical equation is:
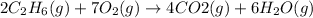
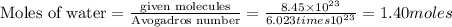
Accoding to stoichiometry:
6 moles of water are produced along with = 4 moles of carbon dioxide
Thus 1.40 moles of water are produced along with =
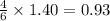 moles of carbon dioxide
moles of carbon dioxide
Molecules of carbon dioxide =