Answer:
Step-by-step explanation:
We are given this formula:
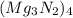
We want to find the number of magnesium atoms. We can see that there is a subscript of 3 after the magnesium, so there are 3 magnesium atoms in 1 molecule of the compound.
However, there is a subscript of 4 that comes after the entire compound, so there are actually 4 molecules, with each molecule containing 3 magnesium atoms. Multiply 4 and 3.
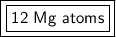
There are 12 magnesium atoms.