Answer:
900 K
Step-by-step explanation:
Recall the ideal gas law:
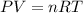
Because only pressure and temperature is changing, we can rearrange the equation as follows:
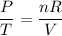
The right-hand side stays constant. Therefore:
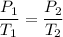
The can explodes at a pressure of 90 atm. The current temperature and pressure is 300 K and 30 atm, respectively.
Substitute and solve for T₂:
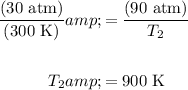
Hence, the temperature must be reach 900 K.