Answer:

Step-by-step explanation:
Given Data:
Mass = m = 96 g = 0.096 kg
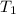 = 124 °C
= 124 °C
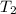 = 158 °C
= 158 °C
Change in Temp. = ΔT = 158 - 124 = 34 °C
Specific Heat Constant = c = 4.186 J/g °C
Required:
Specific Heat Capacity = Q = ?
Formula:
Q = mcΔT
Solution:
Q = (0.096)(4.186)(34)
Q = 13.7 Joules
![\rule[225]{225}{2}](https://img.qammunity.org/2022/formulas/mathematics/high-school/3icqlwn6du2l5ygbr7z2lp6sjjralcpq09.png)
Hope this helped!
~AH1807