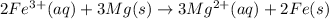Answer:
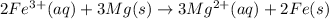
Step-by-step explanation:
Complete ionic equation : In complete ionic equation, all the substance that are strong electrolyte and are present in an aqueous state and represented in the form of ions.
Net ionic equation : In the net ionic equations, we do not not include the spectator ions in the equations.
Spectator ions : The ions present on reactant and product side which do not participate in a reactions. The same ions present on both the sides.
The complete balanced ionic equation will be:
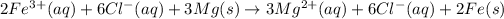
In this equation,
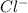 are the spectator ions.
are the spectator ions.
By removing the spectator ions from the balanced ionic equation, we get the net ionic equation.
The net ionic equation will be: