Answer:
The mass of 0.063*10⁻⁴ moles of aluminum sulphate is 2.15*10⁻³ grams.
Step-by-step explanation:
Aluminum sulfate Al₂(SO₄)₃ has a molar mass of 342.15 g/mol.
Molar mass is the amount of mass that a substance contains in one mole of a substance, which can be an element or a compound.
So in this case you can apply the following rule of three: if 342.15 grams are present in 1 mole of aluminum sulfate, how much mass is present in 0.063*10⁻⁴ moles of the compound?
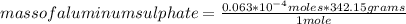
mass of aluminum sulphate= 2.15*10⁻³ grams
The mass of 0.063*10⁻⁴ moles of aluminum sulphate is 2.15*10⁻³ grams.