Answer:
5. The mass of Na₂CO₃, that will produce 5 g of CO₂ is approximately 12.04 grams of Na₂CO₃
6. The mass of nitrogen gas (N₂) that will react completely with 150 g of hydrogen (H₂) in the production of NH₃ is 693.
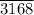 grams of N₂
grams of N₂
Step-by-step explanation:
5. The given equation for the formation of carbon dioxide (CO₂) from sodium bicarbonate (Na₂CO₃) is presented as follows;
(Na₂CO₃) + 2HCl → 2NaCl + CO₂ + H₂O
One mole (105.99 g) of Na₂CO₃ produces 1 mole (44.01 g) of CO₂
The mass, 'x' g of Na₂CO₃, that will produce 5 g of CO₂ is given by the law of definite proportions as follows;
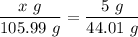
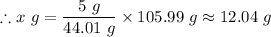
The mass of Na₂CO₃, that will produce 5 g of CO₂, x ≈ 12.04 g
6. The chemical equation for the reaction is presented as follows;
N₂ + 3H₂ → 2NH₃
Therefore, one mole (28.01 g) of nitrogen gas, (N₂), reacts with three moles (3 × 2.02 g) of hydrogen gas (H₂) to produce 2 moles of ammonia (NH₃)
The mass 'x' grams of nitrogen gas (N₂) that will react completely with150 g of hydrogen (H₂) in the production of NH₃ is given as follows;
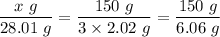
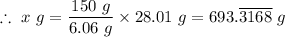
The mass of nitrogen gas (N₂) that will react completely with 150 g of hydrogen (H₂) in the production of NH₃, x = 693.
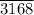 grams
grams