Answer:
The final temperature of both substances at thermal equilibrium is 17.3°C
Step-by-step explanation:
To calculate the final temperature of both substances at thermal equilibrium -:
First , we calculate the heat of A1 cube as follows -
q= mSΔT
(where q = heat of the cube , m = mass of cube , S= specific heat of cube {0.902j/g°C}, T = Temperature )
Putting the values given in the question ,
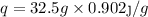 °
°
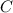
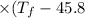 °
°
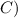
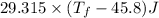
Now , calculate the heat of water -
q=mSΔT
Putting values from the question ,
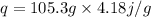 °
°
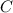
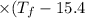 °
°
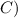
=
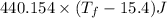
Now ,
Heat lost by water A1= Heat gained by water [negative sign about heat lost]
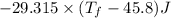
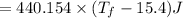
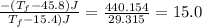
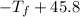 °
°
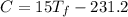 °
°
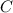
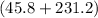 °
°
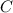 =
=
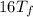
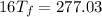 °
°
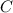
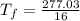 = 17.3°C
= 17.3°C
Therefore , the final temperature of both substances at thermal equilibrium is 17.3°C