Answer:
30.0 mol CO₂
Step-by-step explanation:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
To answer this problem we need to convert moles of C₃H₈ into moles of CO₂: We'll do that by using the stoichiometric coefficients, using a conversion factor that has C₃H₈ moles in the denominator and CO₂ moles in the numerator:
10.0 mol C₃H₈ *
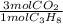 = 30.0 mol CO₂
= 30.0 mol CO₂