Answer:
Since we're sticking with theoretical values instead of experimental, Percent Composition would be (Molar Mass of the element) / (Molar mass of compound) x 100%
Molar mass of Calcium: 40g/mol
Molar mass of Carbon: 12g/mol
Molar mass of Oxygen: 16g/mol
Molar mass of CaCO3 (Calcium Carbonate) = (40) + (12) + (16)*3 = 100g/mol
Percentage composition of Calcium =
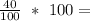 40% of Calcium
40% of Calcium
Percentage composition of Carbon =
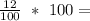 12% of Carbon
12% of Carbon
(For the next one, there are 3 oxygen, so it'd be 16x3 = 48g/mol)
Percentage composition of Oxygen =
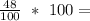 48% of Oxygen
48% of Oxygen