Answer:
Calcium chloride does not have a covalent bond , it is an ionic bond (which means donation of electrons takes place ). The charge of calcium ions is +2, while the charge of sodium ions is -1. The molecule of calcium chloride contains one calcium ion (+2) and two chloride ions (-1), resulting in an overall charge of 0, or neutral.
IONIC BONDING IN CALCIUM CHLORIDE
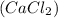
Electron sharing produces covalent compounds, while electron donation produces ionic compounds.
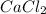 is a salt with an ionic bond. This is because calcium takes up an electron to each of the chlorine atoms, resulting in
is a salt with an ionic bond. This is because calcium takes up an electron to each of the chlorine atoms, resulting in
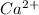 ions for calcium and
ions for calcium and
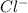 ions for chlorine. At room temperature, it behaves like a normal ionic halide and is solid. Calcium is a metal with a non-metal sulphate bond.
ions for chlorine. At room temperature, it behaves like a normal ionic halide and is solid. Calcium is a metal with a non-metal sulphate bond.
Thus , Calcium chloride have ionic bonds present on them . No covalent bonds takes place in calcium chloride.