Answer:
37.5 mL of a 4.0 M Mg(OH)₂ are needed to make 750 mL of 0.2 M Mg(OH)₂
Step-by-step explanation:
Dilution is the reduction in concentration of a chemical in a solution. Dilution consists of lowering the amount of solute per unit volume of solution.
In other words, dilution is the procedure followed to prepare a less concentrated solution from a more concentrated one, and consists of adding solvent to an existing solution. The quantity or mass of the solute is not changed but only that of the solvent.
In dilutions the expression is used:
Ci*Vi = Cf*Vf
where:
- Ci: initial concentration
- Vi: initial volume
- Cf: final concentration
- Vf: final volume
In this case:
- Ci: 4 M
- Vi: ?
- Cf: 0.2 M
- Vf: 750 mL
Replacing:
4 M* Vi= 0.2 M* 750 mL
Solving:
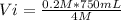
Vi= 37.5 mL
37.5 mL of a 4.0 M Mg(OH)₂ are needed to make 750 mL of 0.2 M Mg(OH)₂